Advancing a broad pipeline of vaccines to prevent infectious diseases.
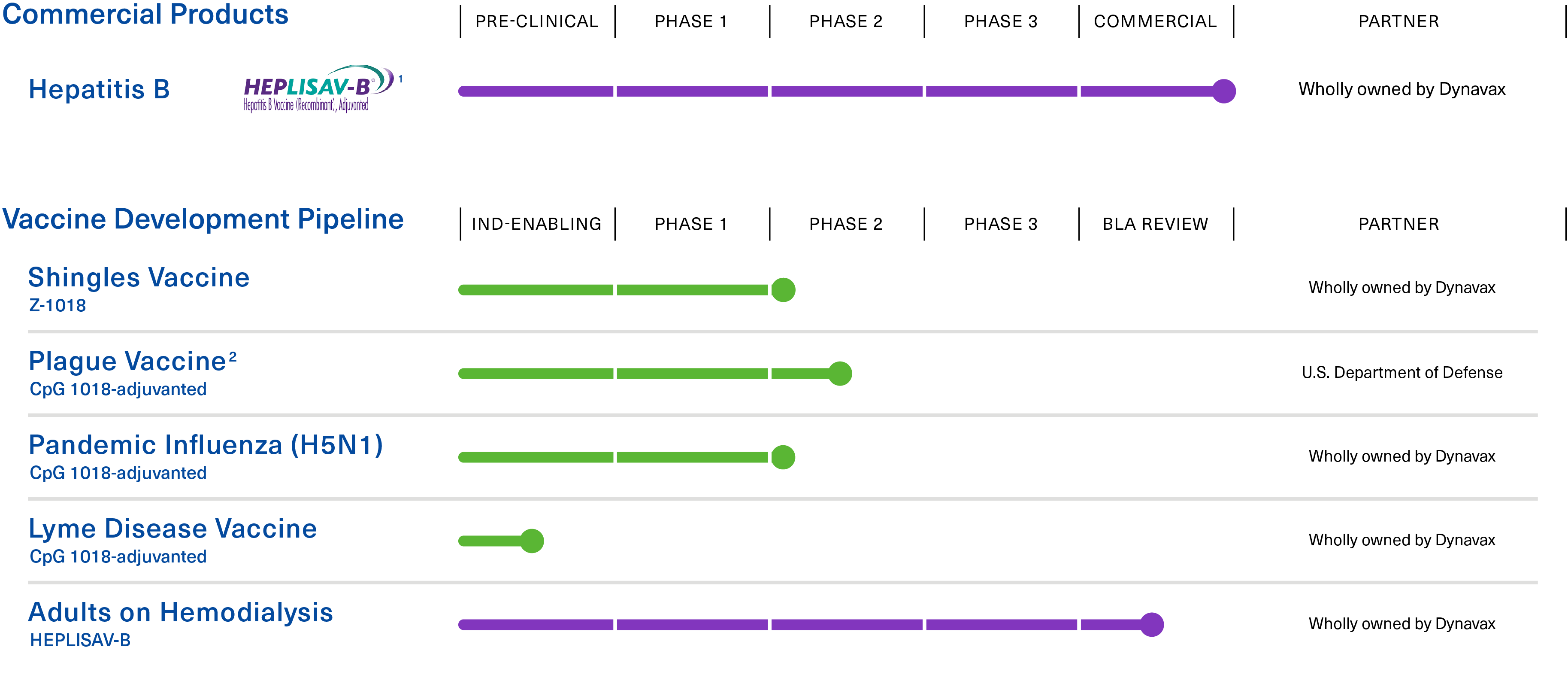
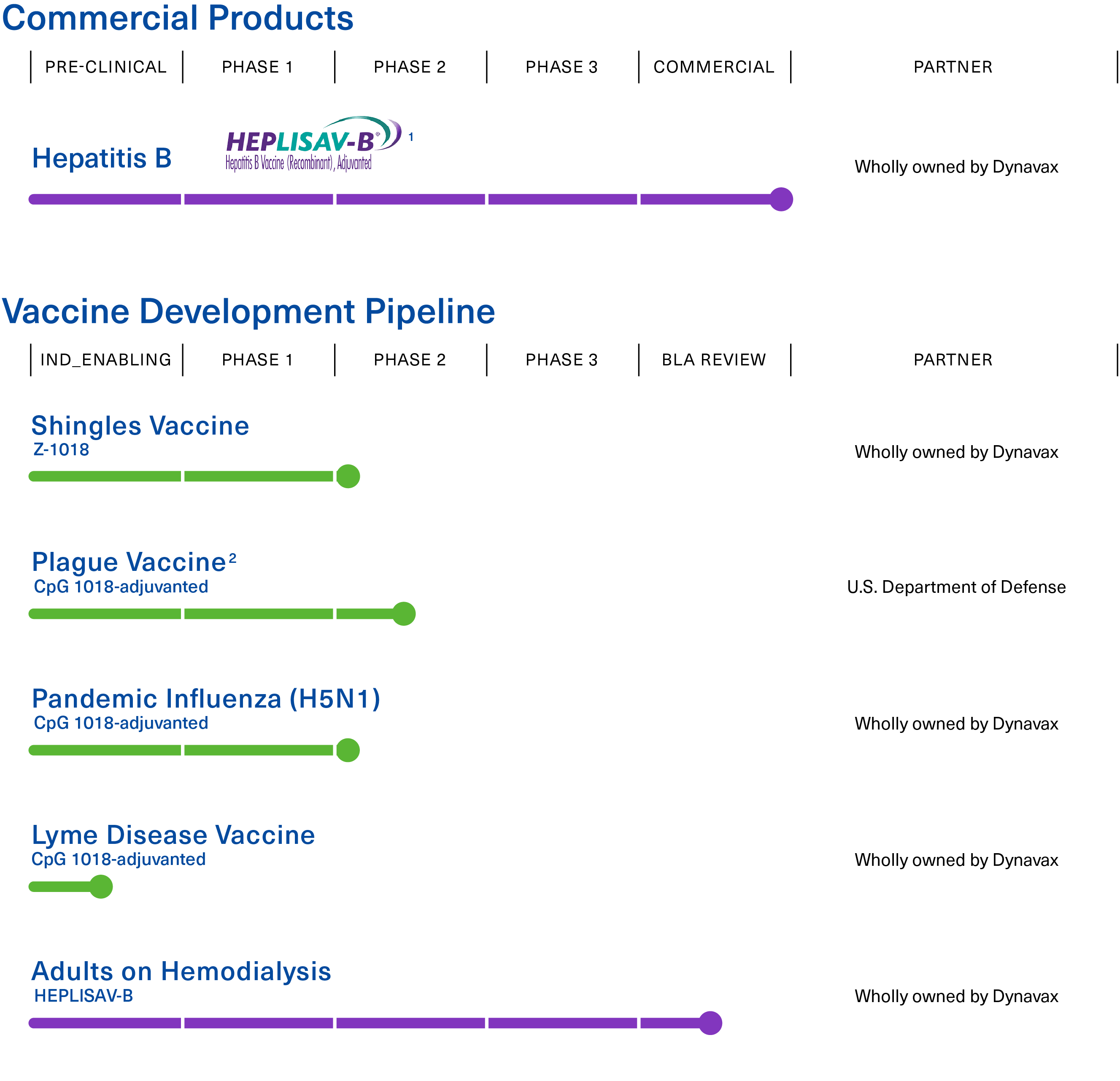
1 APPROVED: U.S. Commercial launch Q1 2018; EU Commercial launch Q2 2022
2 Phase 2 clinical trial to be conducted in collaboration with, and funded by, the U.S. Department of Defense
Clinical Trials
New vaccines that potentially help protect people are only possible because of volunteers who participate in clinical research. Joining a clinical trial is an important decision that should be discussed with a healthcare provider.
DV2-ZOS-02
Overview
A Phase 1/2 randomized, observer-blinded, active-controlled, dose escalation, multicenter trial to evaluate the safety, tolerability, and immunogenicity of an investigational herpes zoster (shingles) vaccine (z-1018) utilizing CpG 1018 adjuvant compared to Shingrix® in approximately 440 healthy adult volunteers between the ages of 50 and 69 years of age.
Status: Recruiting
ClinicalTrials.gov NCT Identifier: NCT06569823
DV2-PLG-01
Overview
A Phase 2, randomized, active-controlled, observer-blind, multicenter trial of the immunogenicity, safety and tolerability of rF1V vaccine with CpG 1018 adjuvant compared with rF1V vaccine alone in approximately 200 healthy volunteers between the ages of 18 and 55 years of age.
Status: Completed
ClinicalTrials.gov NCT Identifier: NCT05506969
The molecules and their uses are investigational, Dynavax has not received approval from any regulatory authority for the use globally, and the safety and efficacy of these molecules has not been established.
